

NeuroActiva™is the early developer of NA-831, which is ready for Phase 3 clinical trials for the prevention and treatment of Alzheimer's disease and other neurodegenerative diseases.

BiomedAI focuses on the application of Artificial intelligence (AI) using computer mimicking “intellectual processes characteristic of humans” to accelerate our drug discovery, drug development and patients screening programs in clinical trials.

Biomed Digital is harnessing the power of wearable technologies and machine learning to create a digital phenotype that helps clinicians to diagnose early onsets of Alzheimer’s disease.

MedAware Systems, Inc. is a next generation medical data and analytics company. Through its Artificial Intelligence applications the Company provides unprecedented business intelligence for drug development, support for regulatory filings, competitive analysis, and marketing messaging.
(http://www.medawaresystems.com)
The management team is led by Dr.Lloyd L. Tran, the company's Chairman and Chief Scientific Officer. Lloyd has been a research scientist with 25 years of experience in drug discovery, working for major pharmaceutical companies including Monsanto and Pfizer.
Biomed has developed Unified Acceleration Platform (UAP), which is based on Dr. Lloyd Tran’s Unified Theory on Neurodegenerative Diseases, Metabolic Disorders, and Cardiovascular Diseases.
The Unified Theory posits that shared molecular, cellular, and systemic dysfunctions—especially in energy metabolism, chronic inflammation, and cellular senescence, neurogenesis —are central drivers of these diseases.
After spending 20 years researching neurodegenerative and metabolic diseases, the Biomed team has developed a family of drugs for the treatment of ALS, Traumatic Brain Injury, Major Depressive Disorder (MDD), Obesity, Stroke, Liver Disease MASJ, and rare diseases including Rett Syndrome and Fragile X.
Biomed's product categories are given below:
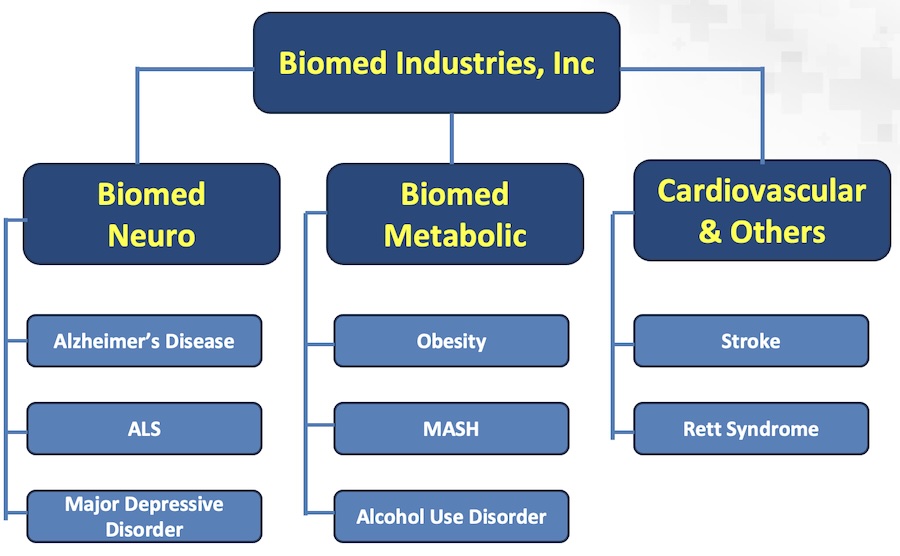
The pipeline of Biomed's products is given below:
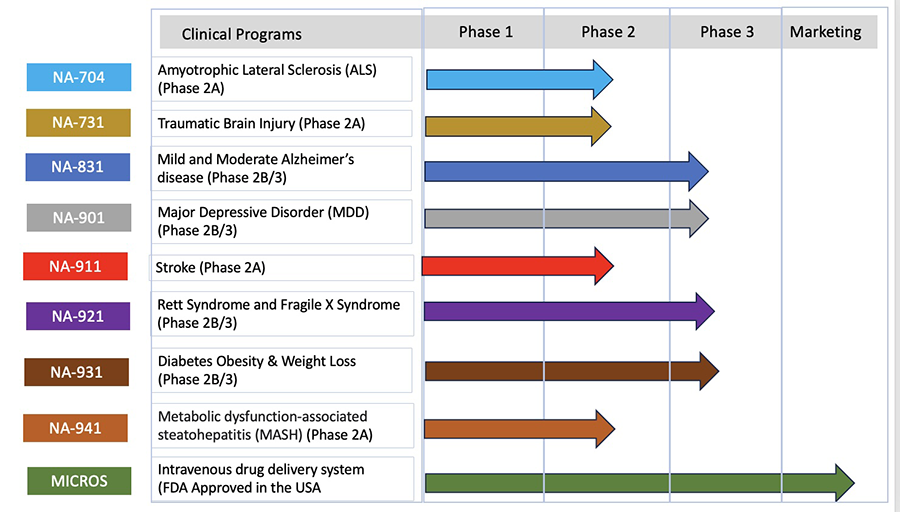
To view our products, please visit our pipeline.
CONTACT: For further information about our products, please contact Mr. Michael Willis, Vice President of Business Development, by completing the Online Contact Form. Thank you.